BioSustane adds abuse-deterrent properties to formulations.
Eastman BioSustane™ SAIB NF is a novel excipient for imparting abuse-deterrent properties to the dosage form. Studies show formulations with BioSustane provide tamper resistance to common forms of tampering, such as crushing and dissolving in liquids. It has been shown that using BioSustane in the formulation makes it difficult to extract the API using commonly available solvents. Crushing the dosage form containing BioSustane results in large-size particles that are difficult to snort. Extraction with alcohol results in a sticky residue that is difficult to handle; even when ingested, there is no observed burst effect.
Experimental matrix using pseudoephedrine hydrochloride (due to its similarity to an opioid)
Ingredients |
Formulations |
|
F1-Control |
F5-Control |
F1 |
F2 |
F3 |
F4 |
F5 |
| Pseudoephedrine HCI | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| BioSustane SAIB NF | –
| –
| 16.78 | 25.17 | 33.56 | 41.95 | 50.34 |
| Polyethylene oxide | 151.02 | 117.46 | 151.02 | 142.63 | 134.24 | 125.85 | 117.46 |
| Tocopherol acetate | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Microcrystalline cellulose | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Magnesium stearate | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Colloidal silicon dioxide | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Total |
219 |
186 |
236 |
236 |
236 |
236 |
236 |
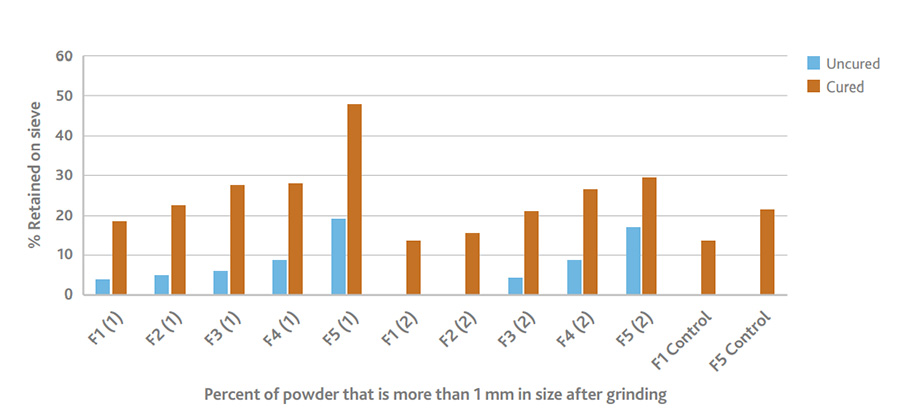
Nasal abuse assessment shows that BioSustane increases the particle size. Up to 47% of the ground powder is >1 mm.
Solvent extraction assessments