BioSustane for amorphous solid dispersion
Highly hydrophobic Eastman BioSustane™ SAIB NF can be used as a carrier for amorphous solid dispersions. This novel excipient does not need hot-melt extrusion or spray-drying manufacturing techniques. Traditional wet granulation techniques can be used to formulate BioSustane amorphous solid dispersions into tablets or capsules. When used as a carrier, BioSustane is shown to improve bioavailability by preventing recrystallization of poorly water-soluble APIs.
The solubility of some poorly water-soluble API in water versus BioSustane.
The solubility of the APIs in BioSustane improves multifold.
APIs
BCS Class II and IV |
Water solubility
(µg/g) |
BioSustane solubility
(µg/g) |
| Rifaximin | 7.1 | 36.36 ± 0.93 x 103 |
| Aripiprazole | 7.7 | 511.6 ± 29.6 x 103 |
| Dolutegravir | 3.176 | 1.7 ± 0.4 x 103 |
| Cyclosporine | 4.0 | 239 ± 12.6 x 103 |
| Tacrolimus | 4.02 | 143.1 ± 28.3 x 103 |
| Sirolimus | 1.73 | 1.92 ± 0.04 x 103 |
| Aprepitant | 3-7 | 0.39 ± 0.04 x 103 |
| Carbamazepine | 152 | 76.54 ± 4.04 x 103 |
BioSustane prevents recrystallization of the poorly water-soluble API. When used as carrier for an amorphous solid dispersion, BioSustane forms a stable dispersion. A study of several weeks of aprepitant formulation with BioSustane through X-ray diffraction revealed no peaks, indicating BioSustane forms a stable, amorphous solid dispersion that inhibits API recrystallization.
BioSustane prevents recrystallization of poorly water-soluble API
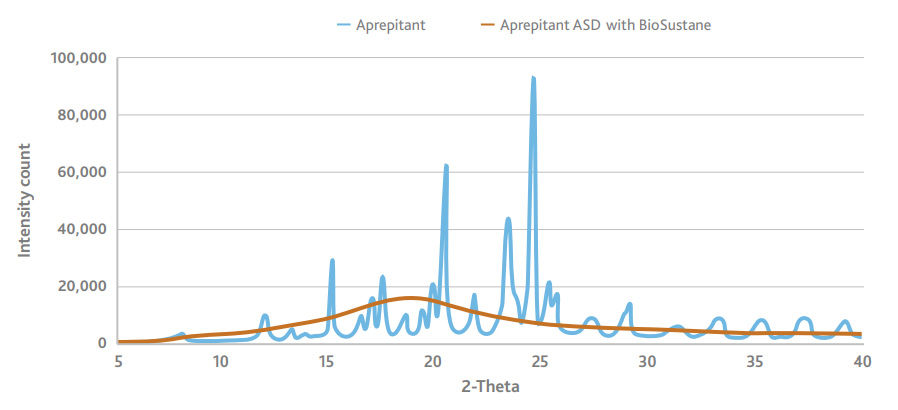
BioSustane is shown to improve bioavailability when used in formulation with other typical excipients. A formulation containing BioSustane and aprepitant, along with a few other excipients, showed two times more bioavailability compared to the formulation without BioSustane. Data of the bioavailability in beagles demonstrates BioSustane can be an effective and novel carrier for drug delivery of poorly water-soluble APIs.
BioSustane improves bioavailability of Aprepitant in beagles
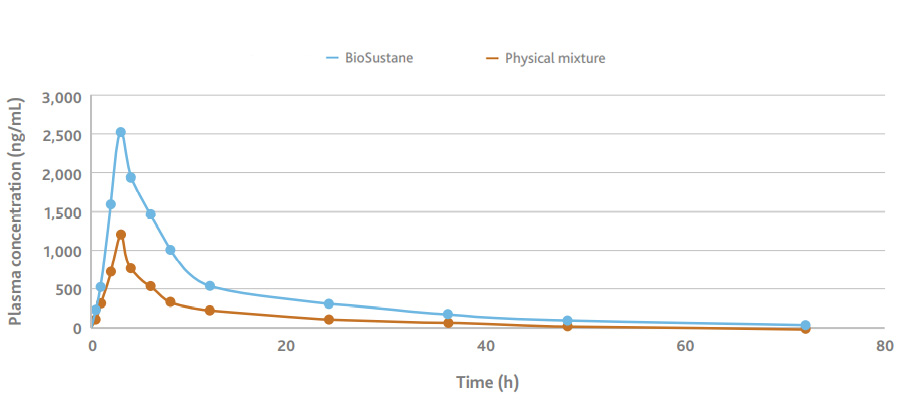
| Pharmacokinetics parameter |
Cmax (ng/mL) |
Tmax(h) |
AUC0–t (ng·h/mL) |
AUC0–∞ (ng·h/mL) |
T1/2 (h) |
Kel (1/h) |
| BioSustane | 2522.9 ± 390.6 | 3 | 25688.4 ± 2692.9 | 26589.6 ± 2624.4 | 13.2 ± 1.6 | 0.053 ± 0.007 |
| Physical mixture | 1214.2 ± 189.6 | 3 | 9784.9 ± 722.1 | 10499.4 ± 437.9 | 14.1 ± 3.3 | 0.05 ± 0.01 |