BioSustane functions as a depot* former for drugs
Depot is usually subcutaneous or intramuscular, depositing the drug in a localized mass (or depot). The drug is gradually absorbed by surrounding tissue. This allows the active compound to be released in a consistent way over a long period of time, improving patient compliance.
BioSustane™ SAIB NF is an excellent lipophilic depot former. It is extensively metabolized in the body into sucrose and partially acylated sucrose. Both are readily absorbed and subsequently eliminated from the body. Release times can vary from a few days to a month or more.
Mechanism of release of API from BioSustane depot
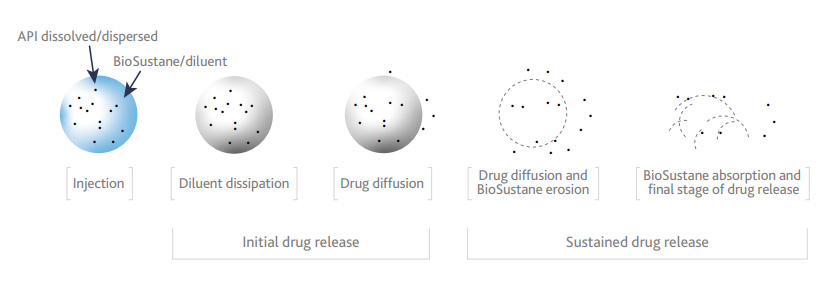
Concept: Consider a solution containing BioSustane, solvent, API and optional additives. The small amount of solvent diffuses into surrounding tissue or interstitial fluid and leaves behind a highly viscous depot. There is delayed release of active ingredient. The breakdown rate of the depot can be slowed through an additive like a biodegradable polymer.