BioSustane enables sustained-release formulations.
Eastman BioSustane™ SAIB NF can be used as a matrix former for sustained release of both hydrophobic (poorly water-soluble) and hydrophilic (water-soluble) APIs. BioSustane is nonpolymeric and does not suffer much from batch-to-batch manufacturing variation, leading to stable product dissolution, which can be difficult to achieve with polymer matrix formers. When used as a sustained-release excipient, BioSustane also imparts abuse deterrence to the dosage form.
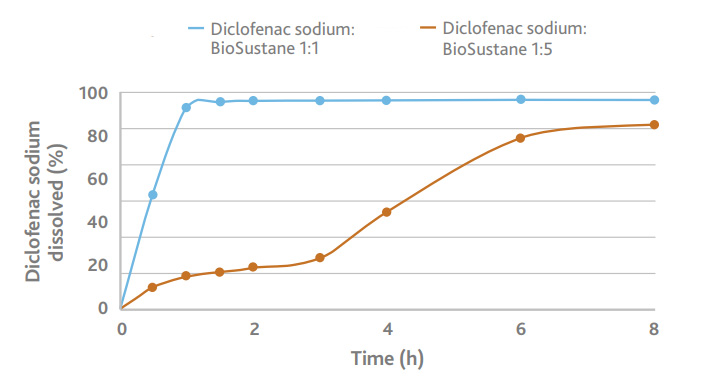
Dissolution of a formulation containing BioSustane, formulated with diclofenac sodium and other excipients.
Sustained release of the API is achieved, and the release can be tuned with varying amount of BioSustane in the formulation.