Dr. Elke von Heimendahl and Dr. Antje Holthausen, Eastman
Pigs are exposed to various types of stress during their lives. There is dietary, social, and environmental stress but also metabolic stress caused by high performance.
In the weaning phase, piglets often show growth depression and are more susceptible to diseases, a phenomenon known as postweaning stress syndrome (Campbell et al., 2013). During this period, social and environmental stresses occur as piglets are separated from their mothers and shifted to new environments. Dietary stress in piglets weaned between three and four weeks of age is a result of replacing easily digestible sow milk with predominantly plant-based solid feed while the digestive tract is still underdeveloped and the barrier function is still incomplete (Moeser et al., 2017). Piglets in the first days after weaning show significantly lower levels of certain vitamins and trace elements—such as vitamin C, alpha- and gamma-tocopherol, and zinc—involved in the oxidative stress response, indicating increased oxidative stress compared to preweaning (Robert et al., 2009). At the same time, a severe increase in pro-inflammatory cytokines such as IL 1ß, IL6, and TNFα can be observed; this is an indication of systemic inflammation (Pie et al., 2004)
The period around weaning is stressful, and supporting piglets with the most suitable diet becomes crucial to minimize any additional impact on the fragile system of the young animals.
The beneficial effect of specialty feed ingredients and additives on gut health as the most promising alternative to antibiotics has become a key topic, increasing antimicrobial resistance (AMR) is one of the world’s most pressing public health issues. Therefore, pro- and metaphylactic use of antimicrobials as well as therapeutical levels of ZnO should be replaced with a holistic approach and products with a focus on adaptive response mechanisms of the entire antioxidant network.
Dietary stress
Dietary stress can be caused by poor-quality feed such as oxidized fat sources, mycotoxins, and antigens in soy—the main plant-based protein source used in piglet diets. This can increase the oxidative stress status of the animal, either directly or indirectly, by triggering inflammation in the gut. The goal should be to prevent local inflammation in the gut and impaired barrier function caused by plant, fungal, and bacterial antigens as well as free radicals. Therefore, the choice of high-quality, well-processed, and hygienic raw materials is very important.
Feed additives to influence digestibility
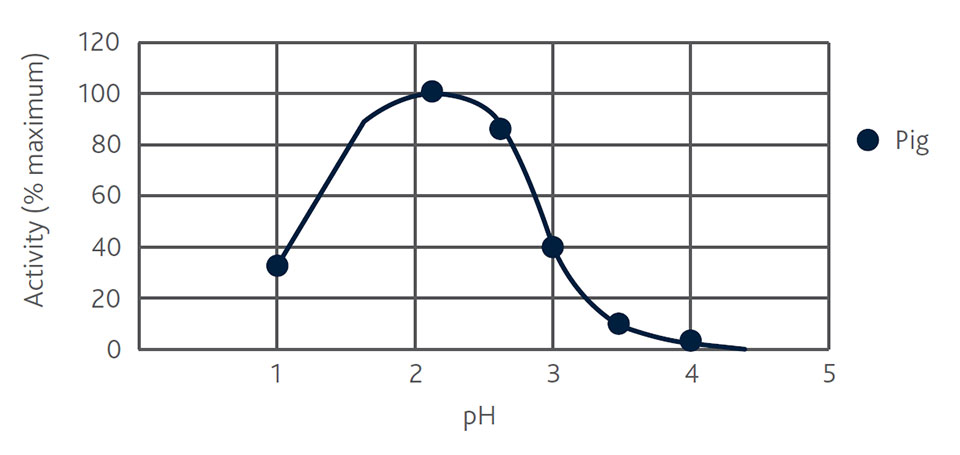
Due to its immature gut system, the piglet’s secretion of digestive enzymes is limited. Specifically, insufficient secretion of pepsin, which is crucial for optimal protein digestion, is a limitation for the digestive capacity in high-performing, young piglets. Undigested protein can reach the hindgut and become a substrate for the growth of pathogenic bacteria, leading to physiological imbalances and serious health issues. Since pepsin has a pH optimum of 2 (Figure 1), organic acids can be used to ensure that this pH optimum in the gastrointestinal tract is met, even in feed with high buffer capacity, such as piglet starter feed.
Figure 1. Activity curve of the protein degrading enzyme pepsin (Crèvieu-Gabriel et al., 1999, adapted)
Short- and medium-chain fatty acids and the effects of gut microflora
Various feed ingredients are known to influence the gut microflora, including prebiotics, probiotics, and organic acids. Beneficial effects on gut health and stress parameters described for probiotics are mainly linked to the modulation of the microbiome directly or indirectly through the production of metabolites such as organic acids—specifically short-chain fatty acids (SCFAs). Organic acids reduce the number of pathogenic bacteria. This way, lipopolysaccharides (LPS), which are an integral part of the outer membrane of gram-negative bacteria such as E. coli and S. enterica and have antigenic properties, are reduced as well. LPS possess pro-inflammatory activities and plays an important role in the pathogenesis of gram-negative bacteria infection.
The growth-inhibiting potential of organic acids on pathogenic bacteria depends on the chain length, the chemical structure and form, and the type of bacteria. Combining different organic acids to modulate the gut microflora and control a broader spectrum of pathogenic bacteria strains is the most effective strategy. However, even a combination of SCFA has limited effect on some critical pathogenic strains of practical relevance, specifically gram-positive strains like S. suis. Further addition of medium-chain fatty acids (MCFAs) could be more beneficial to consider due to their direct growth-inhibiting effect on those bacteria strains. Zentek et al. (2013) reports that the combination of SCFAs and MCFAs on the intestinal microecology in piglets decreasing intestinal pH and the direct reduction of E. coli virulence genes by organic acids could make the combination of SCFAs and MCFAs interesting gut flora modifiers that can eventually prevent postweaning diarrhea.
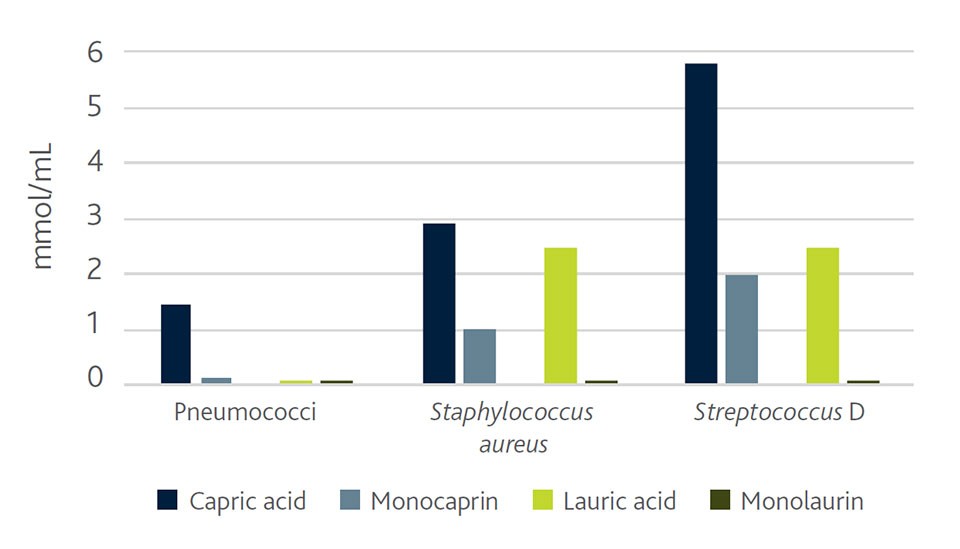
Even more interesting, compared to the usage of pure MCFA, is the inclusion of their monoglycerides. The structural change of the organic acid by esterification with glycerol makes the fatty acid derivative even more effective in inhibiting pathogenic bacteria. This improvement of the inhibitory capacity could be demonstrated on several pathogenic bacteria strains and fatty acids of different chain length (Kabara et al., 1972; Batovska et al., 2009). Figure 2 shows the difference in inhibiting capacity of MCFAs and their monoglyceride derivatives on selected bacteria strains that includes S. suis.
Figure 2. Minimum inhibitory concentration of organic acid derivatives (Kabara et al.,1972; adapted)
Gut development and barrier function
As important as it is to look at reducing pathogens to reduce weaning stress, it is also important to look at ways to improve gut health and barrier function. Butyric acid has received a lot of attention as an efficient alternative to antibiotic treatments due to its broad effects. It is known to have antimicrobial, anti-inflammatory, and antioxidative effects. However, endogenous butyric acid production is limited in the small intestine where there is minimal fiber fermentation. Therefore, addition of butyric acid via feed is necessary to assure continuous availability throughout the digestive tract.
Butyric acid derivatives have demonstrated a clear benefit to intestinal development (Claus et al., 2006). They have been shown to increase the absorption area by increasing the number of plica as well as the number of villi per plica, which is relevant to increase the absorption of nutrients and limit their shift to pathogenic bacteria in the hindgut.
Weaning stress results in long-lasting damage to the gut barrier function, affecting tight junction proteins, which were not recovered 14 days after weaning (Hu et al., 2012). However, trial results with different butyric acid derivatives suggest that their supplementation to diets in periods of stress should be part of a concept against weaning stress.
Immune respons
Literature on butyric acid in humans and animal models indicates the important role it plays in the development of immunity. Butyric acid is able to affect innate as well as adaptive immune cells (Cushing et al., 2015). It has been shown to induce T-cell differentiation (Furusawa et al., 2013) but also to modify macrophage activity against pathogenic bacteria (Schulthess et al., 2019).
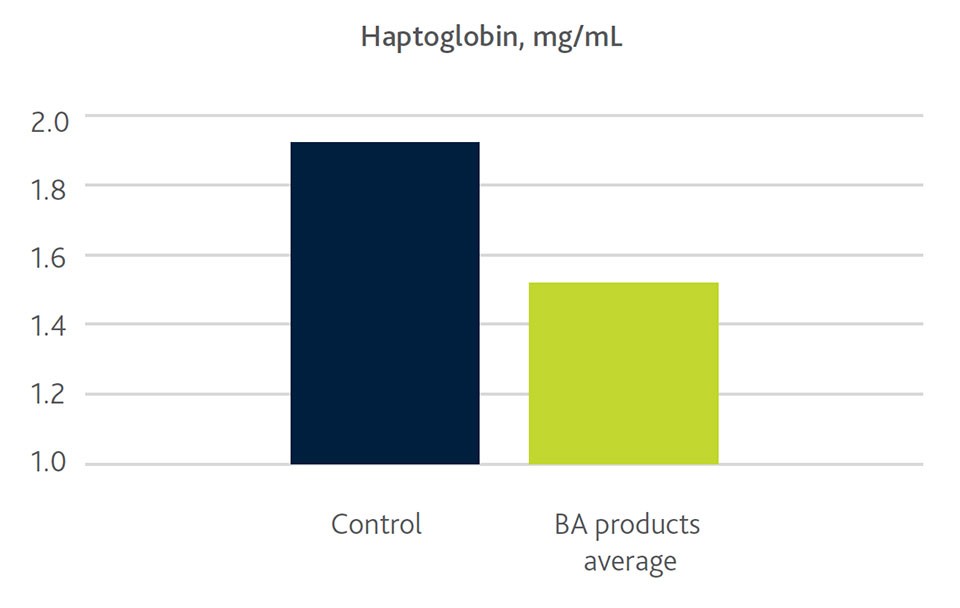
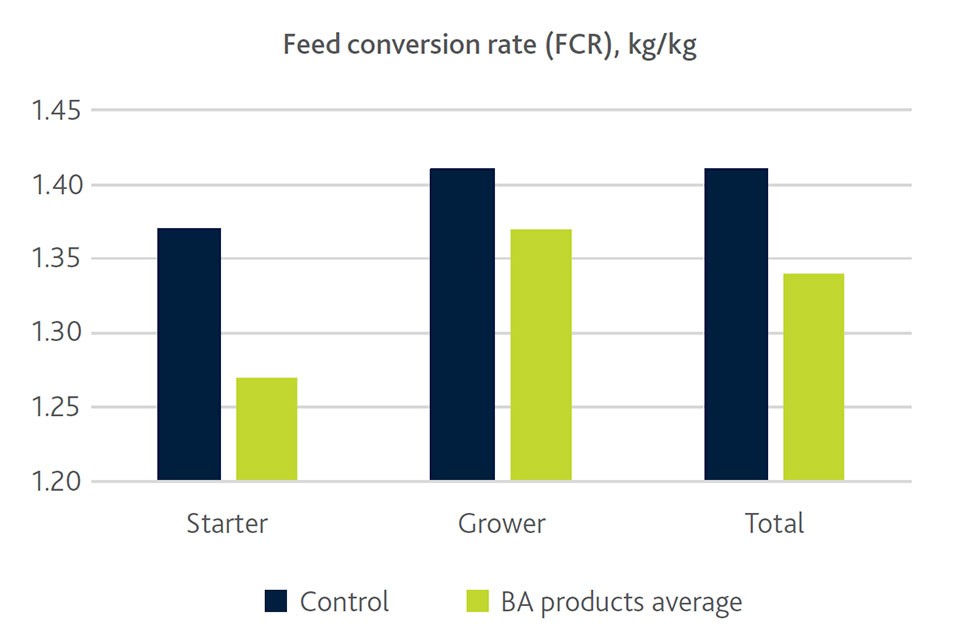
Our own trial results in weaning piglets confirmed significant beneficial effects of butyrate on leucocytes and on the content on the further differentiated monocytes and eosinophils. This is in line with lower levels of the inflammatory marker haptoglobin in supplemented groups, suggesting improved immune function (Figure 3). Less severe inflammation also means less oxidative stress, indicated by higher vitamin E levels which were also observed in butyric acid-supplemented weaning pigs and were still present 41 days after weaning. Reduced stress and a reduced impact on the immune system in the end is also beneficial for performance as indicated by the improved feed conversion ratio in Figure 3.
In conclusion, due to multifactorial problems of weaning stress—including bacterial challenges, oxidative challenges, and inflammation—a holistic concept should be applied to target all aforementioned issues. Therefore, concepts including SCFA, MCFAs, and esters— combined with butyric acid derivatives—seem to be a very helpful tool to combat weaning stress and, at the same time, increase animal performance.
Figure 3. Impact of butyric acid (BA) from various products on inflammatory marker haptoglobin and FCR